Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Ueta, Hirokazu; Fukutani, Katsuyuki*; Yamakawa, Koichiro
Frontiers in Chemistry (Internet), 11, p.1258035_1 - 1258035_7, 2023/08
Times Cited Count:0 Percentile:0.01(Chemistry, Multidisciplinary)Molecular hydrogen has two nuclear-spin modifications called 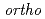 and
and 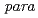 . Because of the symmetry restriction with respect to permutation of the two protons, the
. Because of the symmetry restriction with respect to permutation of the two protons, the 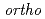 and
and 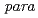 isomers take only odd and even values of the rotational quantum number, respectively. The
isomers take only odd and even values of the rotational quantum number, respectively. The 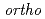 -to-
-to-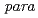 conversion is promoted in condensed systems, to which the excess rotational energy and spin angular momentum are transferred. We review recent studies on fast
conversion is promoted in condensed systems, to which the excess rotational energy and spin angular momentum are transferred. We review recent studies on fast 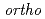 -to-
-to-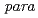 conversion of hydrogen in molecular chemisorption and matrix isolation systems, discussing the conversion mechanism as well as rotational-relaxation pathways.
conversion of hydrogen in molecular chemisorption and matrix isolation systems, discussing the conversion mechanism as well as rotational-relaxation pathways.
Yakushev, A.*; Lens, L.*; D llmann, Ch. E.*; Khuyagbaatar, J.*; J
llmann, Ch. E.*; Khuyagbaatar, J.*; J ger, E.*; Krier, J.*; Runke, J.*; Albers, H. M.*; Asai, Masato; Block, M.*; et al.
ger, E.*; Krier, J.*; Runke, J.*; Albers, H. M.*; Asai, Masato; Block, M.*; et al.
Frontiers in Chemistry (Internet), 10, p.976635_1 - 976635_11, 2022/08
Times Cited Count:11 Percentile:80.03(Chemistry, Multidisciplinary)Flerovium (Fl, element 114) is the heaviest element chemically studied so far. The first chemical experiment on Fl suggested that Fl is a noble-gas-like element, while the second studies suggested that Fl has a volatile-metal-like character. To obtain more reliable conclusion, we performed further experimental studies on Fl adsorption behavior on Si oxide and gold surfaces. The present results suggest that Fl is highly volatile and less reactive than the volatile metal, Hg, but has higher reactivity than the noble gas, Rn.
Yakushev, A.*; Lens, L.*; D llmann, C. E.*; Block, M.*; Nagame, Yuichiro*; Sato, Tetsuya; Toyoshima, Atsushi*; 42 of others*
llmann, C. E.*; Block, M.*; Nagame, Yuichiro*; Sato, Tetsuya; Toyoshima, Atsushi*; 42 of others*
Frontiers in Chemistry (Internet), 9, p.753738_1 - 753738_9, 2021/11
Times Cited Count:11 Percentile:62.25(Chemistry, Multidisciplinary)Nihonium (Nh, element 113) and flerovium (Fl, element 114) are the first superheavy elements in which the 7p shell is occupied. High volatility and inertness were predicted for Fl due to the strong relativistic stabilization of the closed 7p sub-shell, which originates from a large spin-orbit splitting between the 7p
sub-shell, which originates from a large spin-orbit splitting between the 7p and 7p
and 7p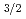 orbitals. One unpaired electron in the outermost 7p
orbitals. One unpaired electron in the outermost 7p sub-shell in Nh is expected to give rise to a higher chemical reactivity. Theoretical predictions of Nh reactivity are discussed, along with results of the first experimental attempts to study Nh chemistry in the gas phase. The experimental observations verify a higher chemical reactivity of Nh atoms compared to its neighbor Fl and call for the development of advanced setups. First tests of a newly developed detection device miniCOMPACT with highly reactive Fr isotopes assure that effective chemical studies of Nh are within reach.
sub-shell in Nh is expected to give rise to a higher chemical reactivity. Theoretical predictions of Nh reactivity are discussed, along with results of the first experimental attempts to study Nh chemistry in the gas phase. The experimental observations verify a higher chemical reactivity of Nh atoms compared to its neighbor Fl and call for the development of advanced setups. First tests of a newly developed detection device miniCOMPACT with highly reactive Fr isotopes assure that effective chemical studies of Nh are within reach.
Nakagawa, Hiroshi; Tamada, Taro*
Frontiers in Chemistry (Internet), 9, p.738077_1 - 738077_7, 2021/10
Times Cited Count:10 Percentile:58.45(Chemistry, Multidisciplinary)Protein hydration is crucial for the stability and molecular recognition of a protein. Water molecules interact with a protein surface via hydrogen bonding. Here, we examined the hydration structure and hydrogen bonding state of a globular protein, staphylococcal nuclease, at various hydration levels in a crystalline state by all-atom molecular dynamics simulation. The hydrophobic residue surface was found to be more hydrated than the hydrophilic residue surface, but both were uniformly hydrated in response to increased water content. In addition, the hydrogen bonds in hydrated water have a tetrahedral structure, which is not much different from the structure of bulk water. The hydrogen bonding structure is compatible with the results of neutron crystallography. The simulations are useful for analyzing the hydration structure and hydrogen bonding state in the crystalline state, and will greatly assist in the further analysis of the information obtained from crystal structure analysis.
Nakagawa, Hiroshi; Oyama, Taiji*
Frontiers in Chemistry (Internet), 7, p.731_1 - 731_9, 2019/11
Times Cited Count:37 Percentile:80.99(Chemistry, Multidisciplinary)Water activity is thermodynamic value defined as the ratio of the equilibrated vapor pressure of the sample to the vapor pressure of pure water at the same temperature. It is known that this value is a reliable indication of the microbial growth, enzymatic activity, and preservation and quality of foods. However, a molecular basis of water activity is still under debate in the related multiple area. Glycerol-water mixtures can provide the variation of water activities by controlling the ratio of glycerol and water. In this study, molecular basis of water activity was examined by using differential scanning calorimetry, attenuated total reflection Fourier-transform infrared spectroscopy and incoherent quasi-elastic neutron scattering based on isotherm sorption curve of glycerol-water mixtures. We found that diffusive dynamics of water is correlated with hydrogen bond related local vibrational dynamics in the glycerol-water mixtures. More importantly, water activity of glycerol-water mixtures can be explained by the hydrogen boding network and molecular dynamics of water in the solutions.